Presented by:┬Ā Dr. Andrew D. Schwarz & Liban M. A. Saleh
Research Leader:┬Ā Prof. Philip Mountford & Prof. Simon Aldridge
Published:┬Ā Journal of the American Chemical Society
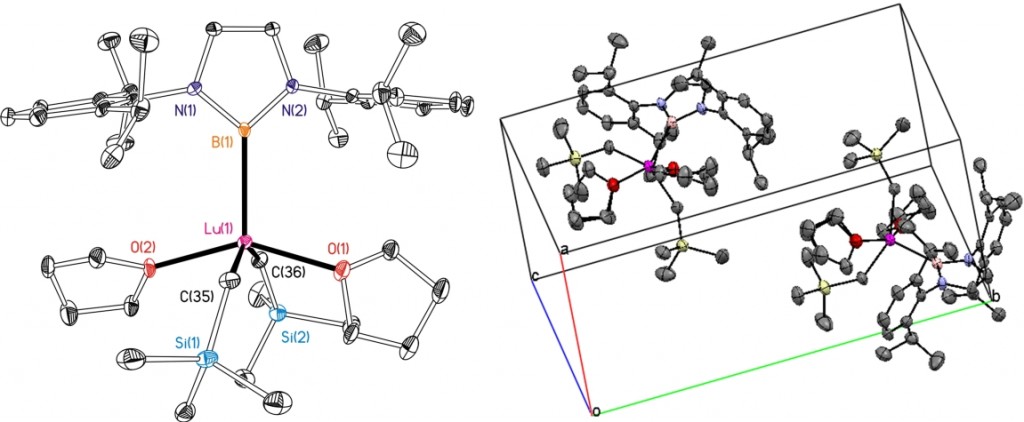
Transition-metal boryl compounds (L)M(BX2)x, containing 2ŌĆæcenter, 2ŌĆæelectron ŽāŌĆæbonds have been a topic of outstanding interest due to pivotal roles in a variety of catalytic and stoichiometric transformations, e.g. hydroboration and diboration of CŌĆōC ŽĆ-bonds, and functionalization of alkane and arene CŌĆæH bonds. To date, virtually all boryl complexes have been prepared either by BŌĆæX (X┬Ā=┬ĀH or halogen) or BŌĆæB oxidative addition to a low oxidation state (L)M species, or by nucleophilic attack of a [(L)M]ŌĆæ anion on a XBR2 or related source of the boryl moiety. However, utilisation of the nucleophilic Li{B(NArCH)2}(THF)2 (Ar┬Ā=┬Ā2,6ŌĆæC6H3iPr2), allows access to rare earth metal boryl compounds.┬Ā Data were collected on a small colourless crystal (0.05┬Ā├Ś┬Ā0.05┬Ā├Ś┬Ā0.05┬Āmm) using the new Oxford Diffraction (Agilent) SuperNova diffractometers and copper radiation.