The results have just been announced for the latest round of applications to the John Fell Oxford University Research Fund. Our proposal for funding for a temperature controlled Peltier stage microscope with polarizing filters for the study of solid-state phase transitions in crystalline materials was successful. Polarized light allows the ready detection of discontinuous changes in the anisotropy of a material during heating or cooling since discontinuity in any property is symptomatic of a phase change (an order-disorder transformation, a change in crystal symmetry, or the formation of a completely new phase). Studying such phase changes using a temperature controlled stage can assist in optimising conditions for obtaining a given form of a material, and also leading to an understanding the nature or mechanism of the transformation.
Presented by: Nicholas G. White & Dr. Fabiola Zapata
Research Leader: Prof. Paul D. Beer
Published: Journal of the American Chemical Society
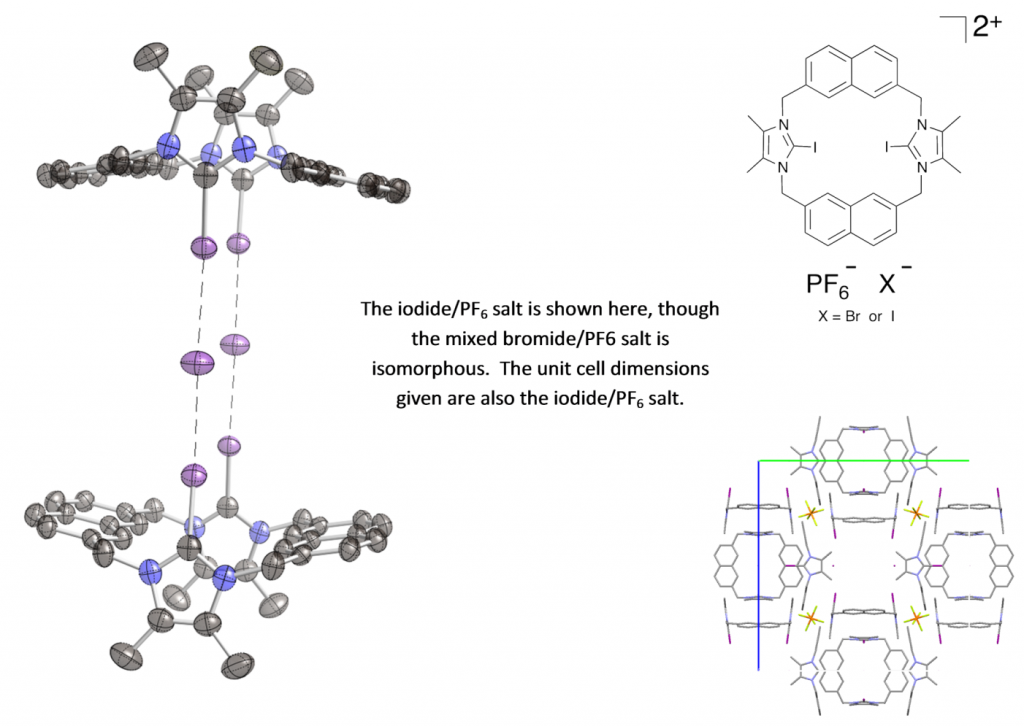
Both the mixed bromide/PF6 and mixed iodide/PF6 salt of this bis-iodoimidazolium macrocycle crystallize in the unusual cubic spacegroup I m -3 (there are currently only six organic structures in this spacegroup in the CSD). The macrocycle binds anions strongly and selectively in competitive methanol/water solvent mixtures, with anions being bound solely by halogen-bonding interactions. In the solid state, the both the bromide and iodide salts of the macrocycle exist as a dimer, with two macrocycles each pointing at two halide anions. Experiments are being undertaken to see if this arrangement occurs in solution.
 Andrew and his research group are close associates of Chem. Cryst.. We share group meetings and, although they have some very strange ideas about what constitutes a single crystal, they collect data in the lab.
Andrew and his research group are close associates of Chem. Cryst.. We share group meetings and, although they have some very strange ideas about what constitutes a single crystal, they collect data in the lab.
For more information, see their webpage.
 Keith Prout died quite suddenly in September 2007 after a short illness.
Keith Prout died quite suddenly in September 2007 after a short illness.
Although he had been officially retired for several years, he was continuing an active research program with Nick Rees combining X-ray and NMR studies. X-ray structure analysis gives information about disorder and thermal vibrations in crystals. When combined with solid state NMR a complete dynamic profile of the crystal can be obtained.
Previous studies of penicillins, ansa-titanocenes and tri-ethyl phosphate molecular complexes with various hosts suggest a variety of molecular motions in addition to the ‘translation-libration-screw’ motion derived from the crystallographic atomic displacement parameters. He also studied order/disorder phase transitions, molecular motion and chiral selectivity in deoxycholic acid molecular complexes.
 James is mathematician by training, who became a a particle physicist and now applies his skills to understanding some of the problems associated with errors in least-squares refinement. He is working on programming an error analysis library.
James is mathematician by training, who became a a particle physicist and now applies his skills to understanding some of the problems associated with errors in least-squares refinement. He is working on programming an error analysis library.
Mustapha is a Senior Research Associate in the group and is a highly experienced and efficient programmer. His specialism is C++ and he is responsible for the Small Molecule ToolKit, SMTK.
 Violeta is an assistant professor in the Chemistry Department at the University of Novi Sad, Serbia. She obtained her Ph.D. in coordination chemistry studying the synthesis, structure and biological activity of transition metal compounds and visited for a few months to learn more about crystallography.
Violeta is an assistant professor in the Chemistry Department at the University of Novi Sad, Serbia. She obtained her Ph.D. in coordination chemistry studying the synthesis, structure and biological activity of transition metal compounds and visited for a few months to learn more about crystallography.
 Terry worked for the Atomic Energy Research Establishment, Harwell for many years before he joined Chem. Cryst.
Terry worked for the Atomic Energy Research Establishment, Harwell for many years before he joined Chem. Cryst.
Terry was one of the pioneers of the use of neutron scattering in the United Kingdom and a founder member of the BCA. Although interested in many areas of crystallography, his particular specialities are neutron scattering studies of crystal structures and lattice dynamics of inorganic materials. Recent interests are the determination of the crystal structures of the oxides of uranium and the nature of the phase transitions in U4O9. The oxide studies are undertaken in collaboration with Frederico Garrido of the University of Paris, Lech Nowicki of the Soltan Institute of Nuclear Physics, Warsaw and Alberto Albinati of the University of Milan.
The author of a number of highly regarded books, there is a prize awarded in his honour sponsored by the Institute of Physics and the Royal Society of Chemistry.
 Emma’s research is focussed on developing a better understanding of the solid state. She is using a three pronged attack, studying the crystallisation of chalcones; examining the effect of temperature on a material that undergoes a phase transition, and investigating the World’s Favourite Space Group, P21/c. She is using a wide range of probes, including the Cambridge Structural Database, dSNAP, Laue Diffraction, Solid State NMR and Variable Temperature Single Crystal Diffraction as well as collecting data at Diamond. When not fighting to grow crystals or preparing dinner for the team at Diamond, she enjoys dancing, but refuses to perform for the group.
Emma’s research is focussed on developing a better understanding of the solid state. She is using a three pronged attack, studying the crystallisation of chalcones; examining the effect of temperature on a material that undergoes a phase transition, and investigating the World’s Favourite Space Group, P21/c. She is using a wide range of probes, including the Cambridge Structural Database, dSNAP, Laue Diffraction, Solid State NMR and Variable Temperature Single Crystal Diffraction as well as collecting data at Diamond. When not fighting to grow crystals or preparing dinner for the team at Diamond, she enjoys dancing, but refuses to perform for the group.