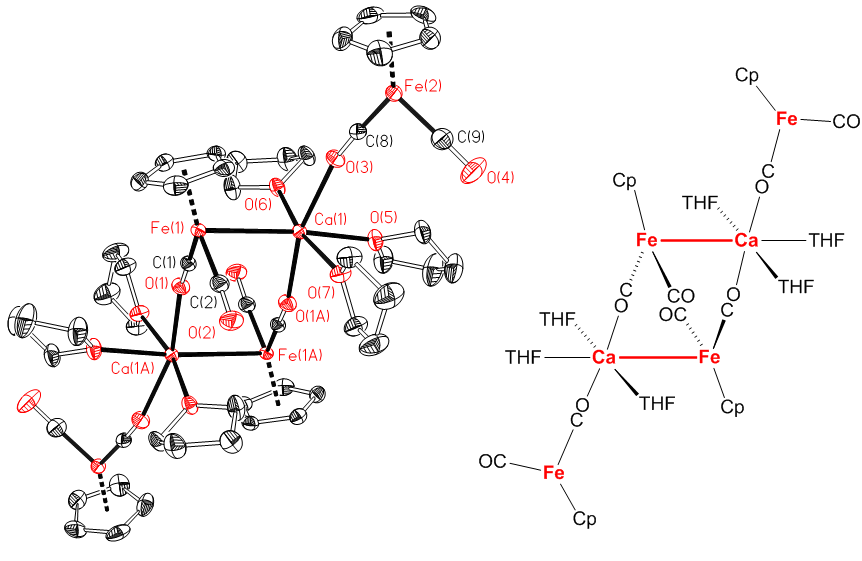
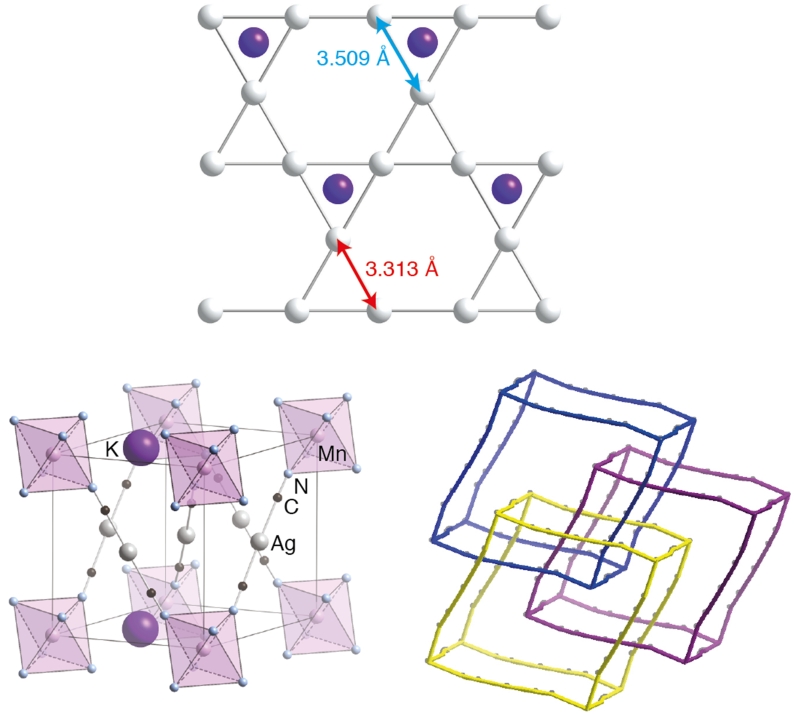
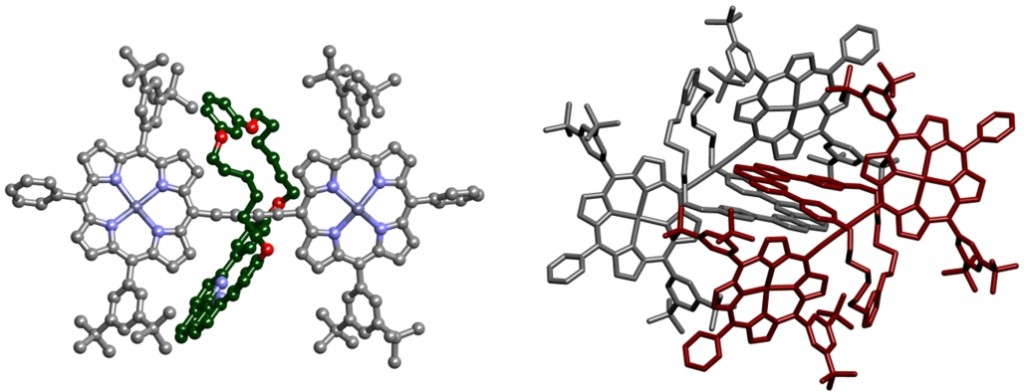
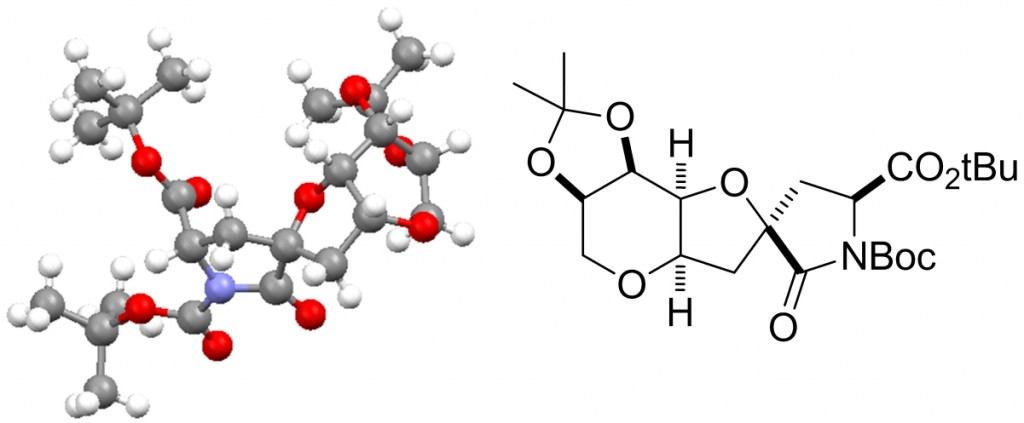
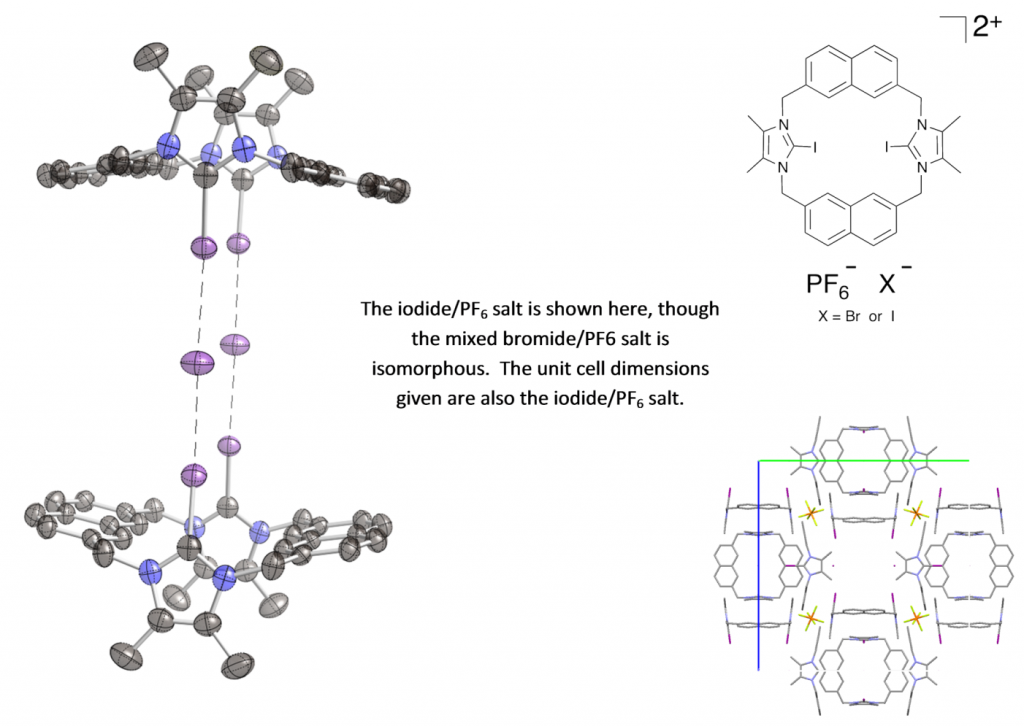
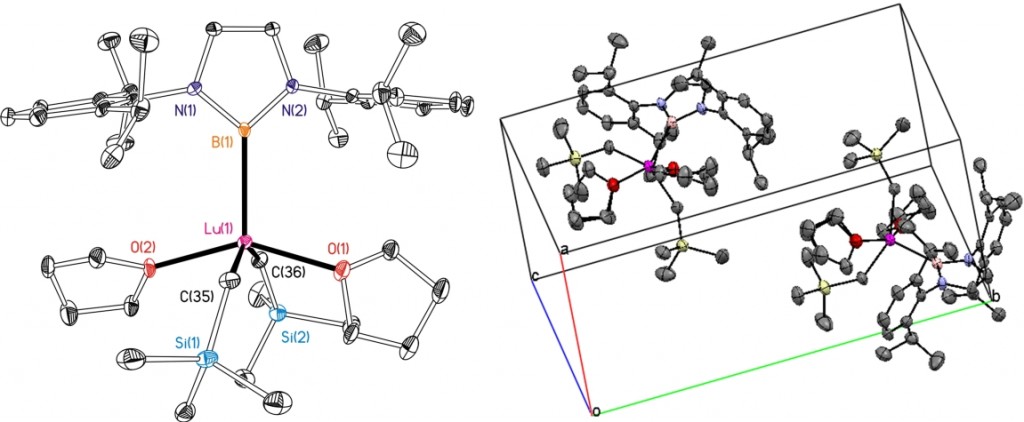
Presented by: Vanessa E. Fairbank & Dr. Amber L. Thompson
Research Leader: Dr. Andrew L. Goodwin
Published: Physical Review B
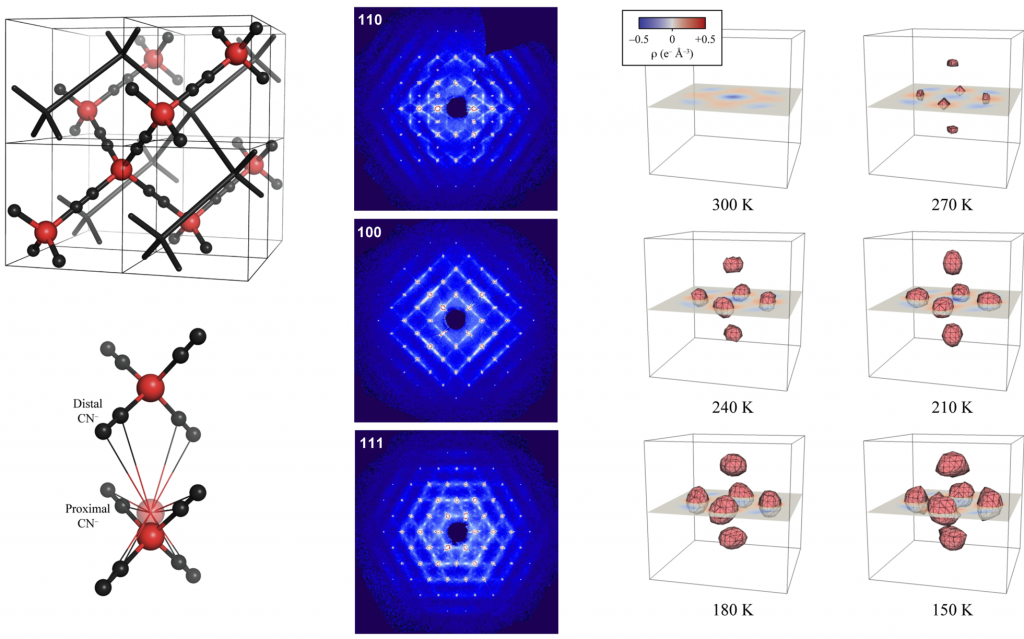
Cubic Cd(CN)2 shows the strongest known isotropic negative thermal expansion (NTE; volume contraction on heating).┬Ā Variable-temperature single-crystal XŌĆæray diffraction suggests there is temperature-dependent off-centering of Cd2+ ions that has the effect of increasing the cadmium coordination volume at low temperatures, providing an alternate mechanism for NTE in this material. These displacements are evident in the residual electron density and the highly-structured diffuse scattering in the experimental X-ray diffraction patterns.┬Ā Using Monte Carlo simulations, we have interpreted these patterns in terms of a basic set of ŌĆ£ice-rulesŌĆØ that establish a mapping between the dynamics of Cd(CN)2and proton ordering in cubic ice VII.