The 2016 British Crystallographic Meeting Spring Meeting took place at the University of Nottingham from 4th – 7th April. Contributions from Chem. Cryst. staff and students were:
The 2016 British Crystallographic Meeting Spring Meeting took place at the University of Nottingham from 4th – 7th April. Contributions from Chem. Cryst. staff and students were:
Jerome G. P. Wicker, Bill I. F. David & Richard I. Cooper
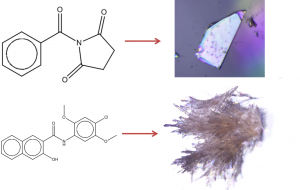
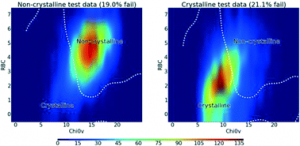
When will it Crystallise? (Talk in session: From Amorphous to Crystal)
Jo Baker & Richard I. Cooper
Making and Measuring Photoswitchable Materials (Talk in session: Young Crystallographers’ Satellite)
Pascal Parois, Karim J. Sutton & Richard I. Cooper
On the application of leverage analysis to parameter precision using area detector strategies (Poster)
Oliver Robshaw & Richard I. Cooper
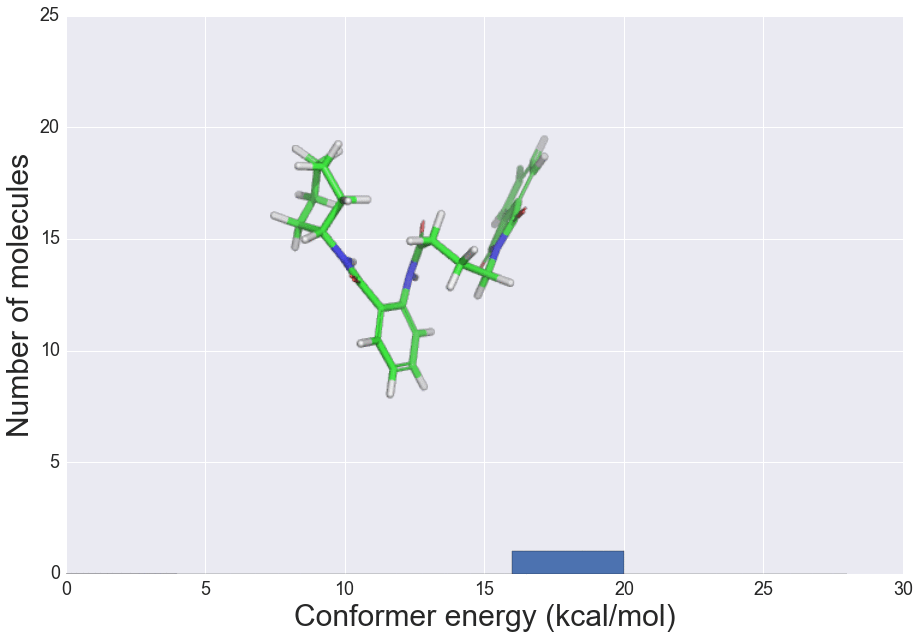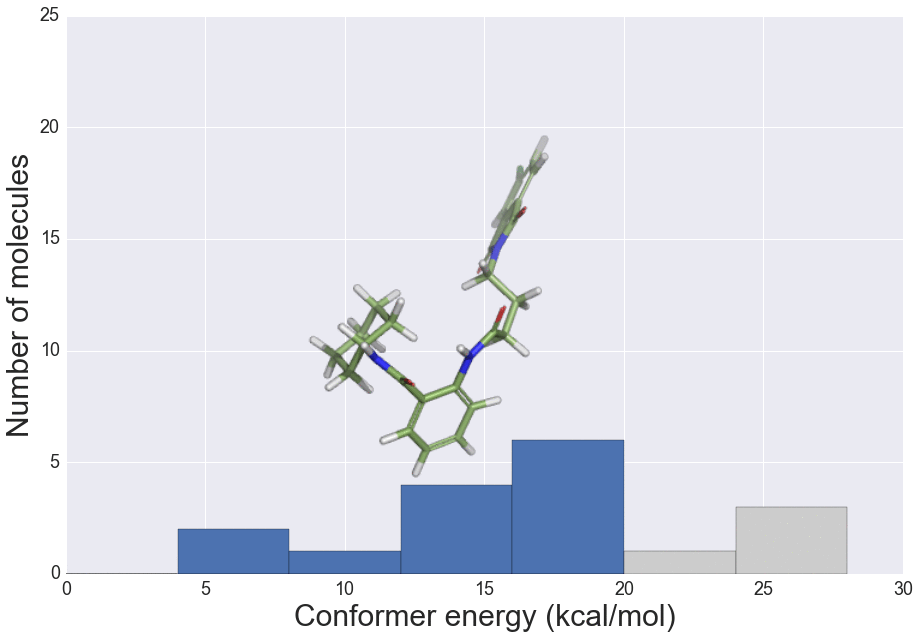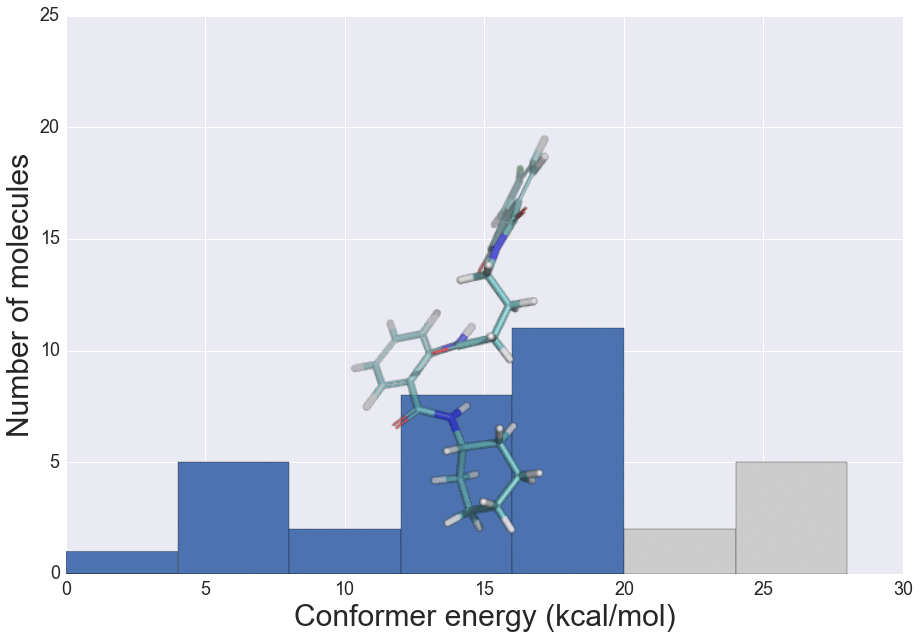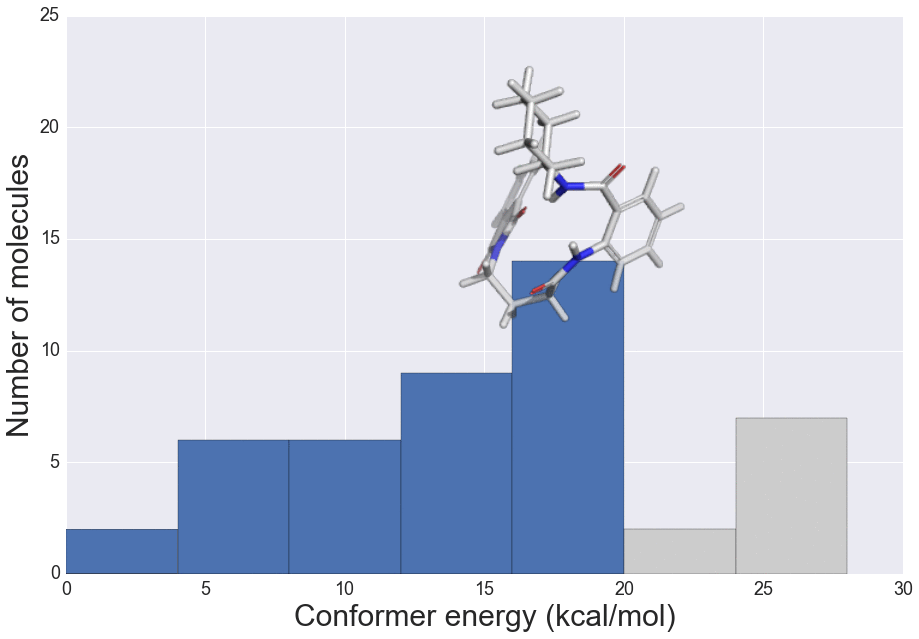
The role of molecular similarity in crystal structure packing (Poster)
Katie McInally & Richard I. Cooper
Linking crystallization prediction, theory and experiment using solubility curve determination (Poster)
Richard I. Cooper, Pascal Parois & David J. Watkin
Non-routine single crystal structure analyses using CRYSTALS (Poster)
Alex Mercer & Richard I. Cooper
Fitting Disordered Crystal Structures by Simulated Annealing of an Ensemble Model (Poster)