Presented by:Â Nicola K. S. Davis & Dr. Amber L. Thompson
Research Leader:Â Prof. Harry L. Anderson
Published:Â Journal of the American Chemical Society
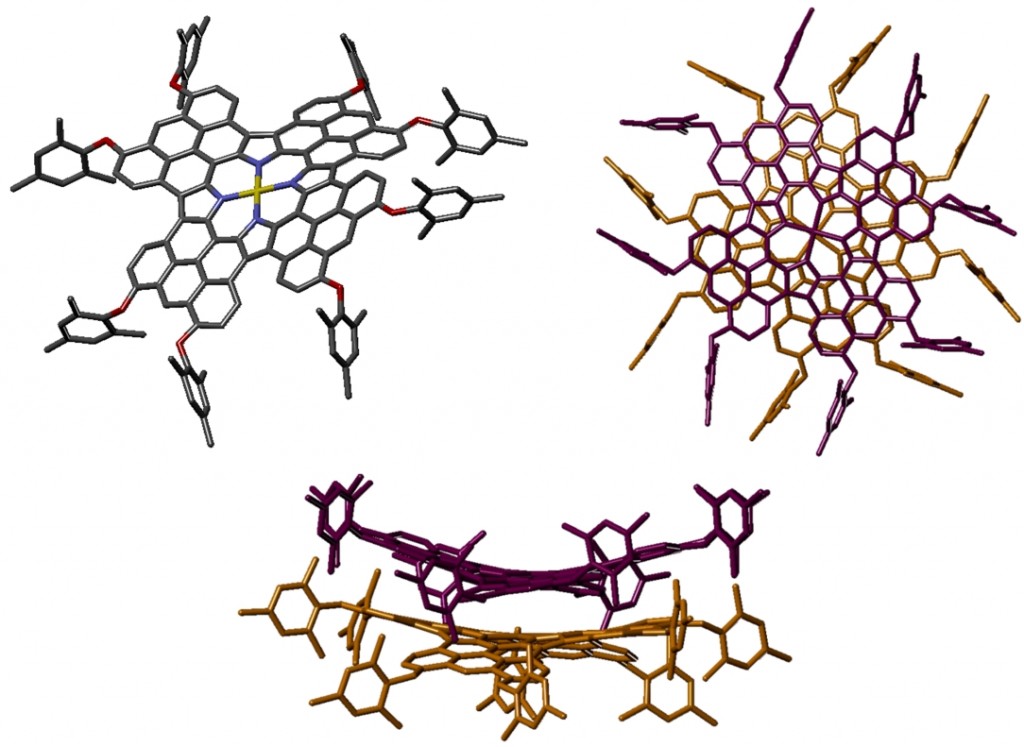
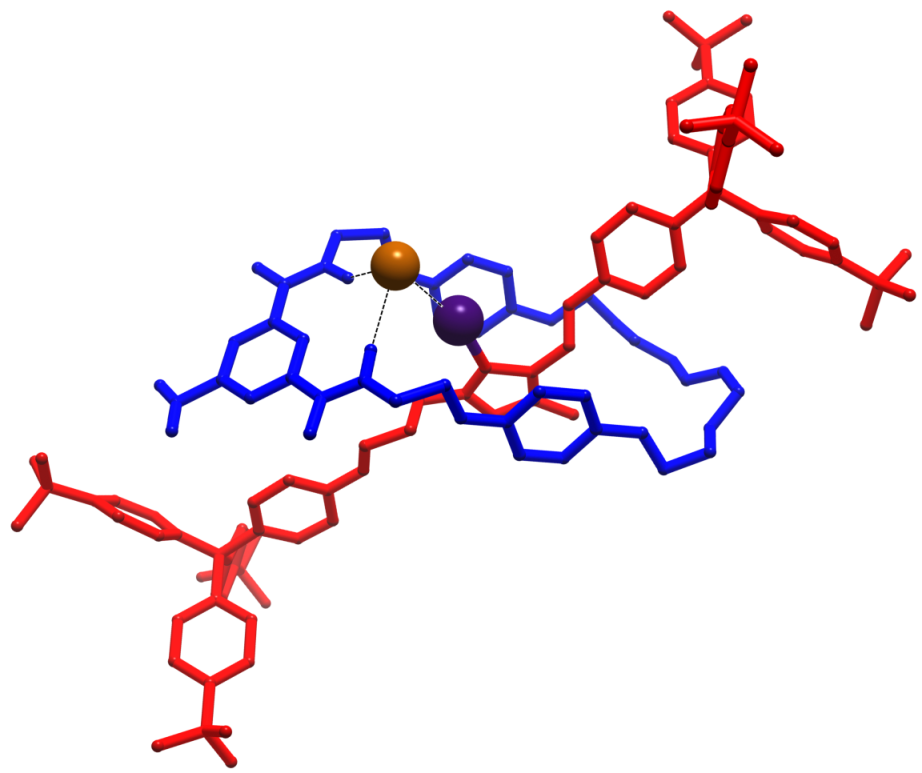
Molecules with large planar π-systems show a strong tendancy to aggregate due to π-π interactions. This tetra-anthracene-fused porphyrin forms dimers with the molecules twisted with respect to each other. Bulky aryl groups were necessary for characterisation, but prevent the porphyrins from forming longer stacks in the crystal. Using long alkyl chains instead could yield systems which form longer π-stacked arrays which may form discotic liquid crystals. Furthermore, as the porphyrins stack with a near-zero horizontal offset, these have potential as light harvesting arrays since the alignment of the chromophores provides an efficient pathway for holes and electrons along the column.