Journal of the American Chemical Society 2016, 138(40), 13314-13325 [doi:10.1021/jacs.6b07501]
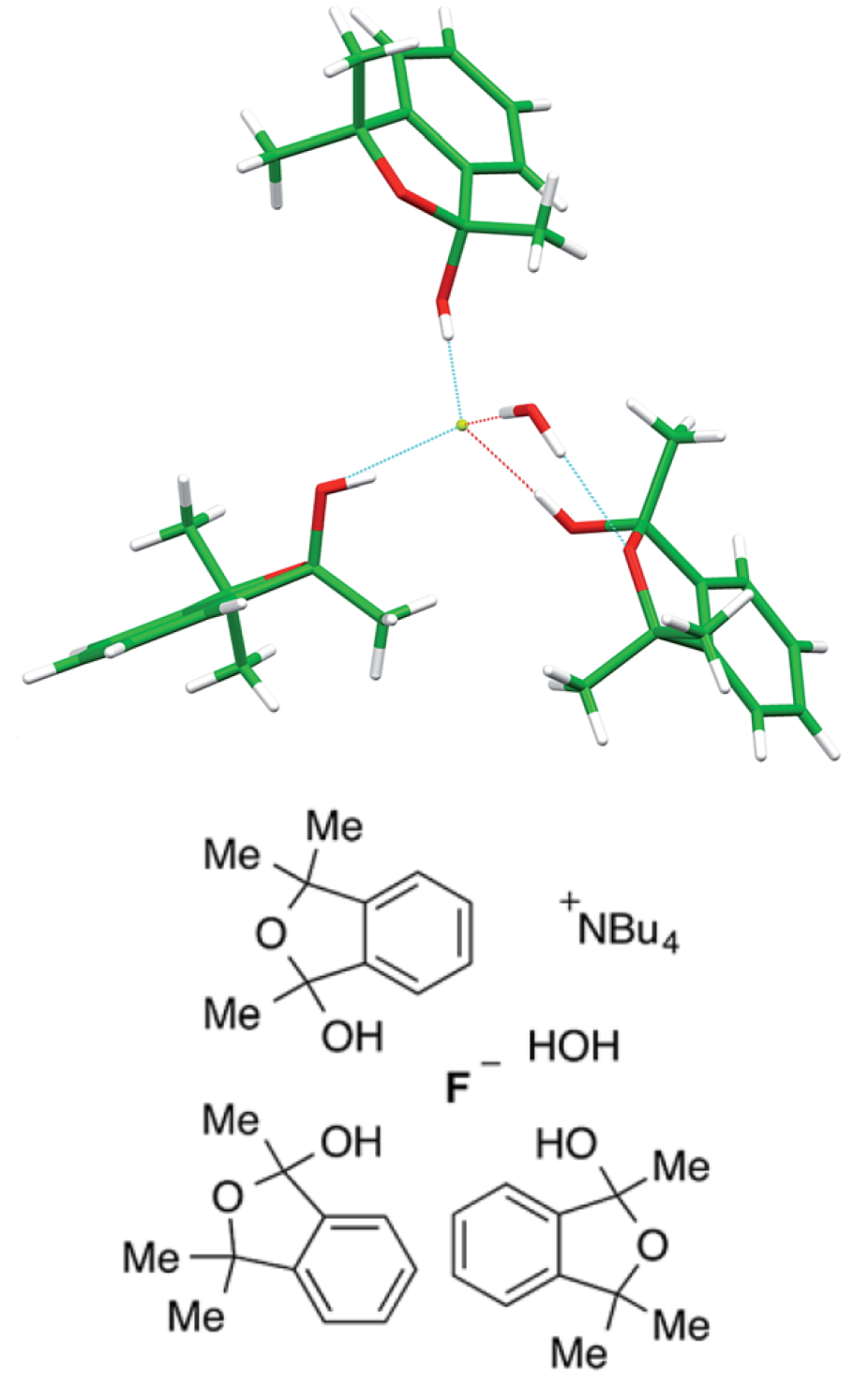
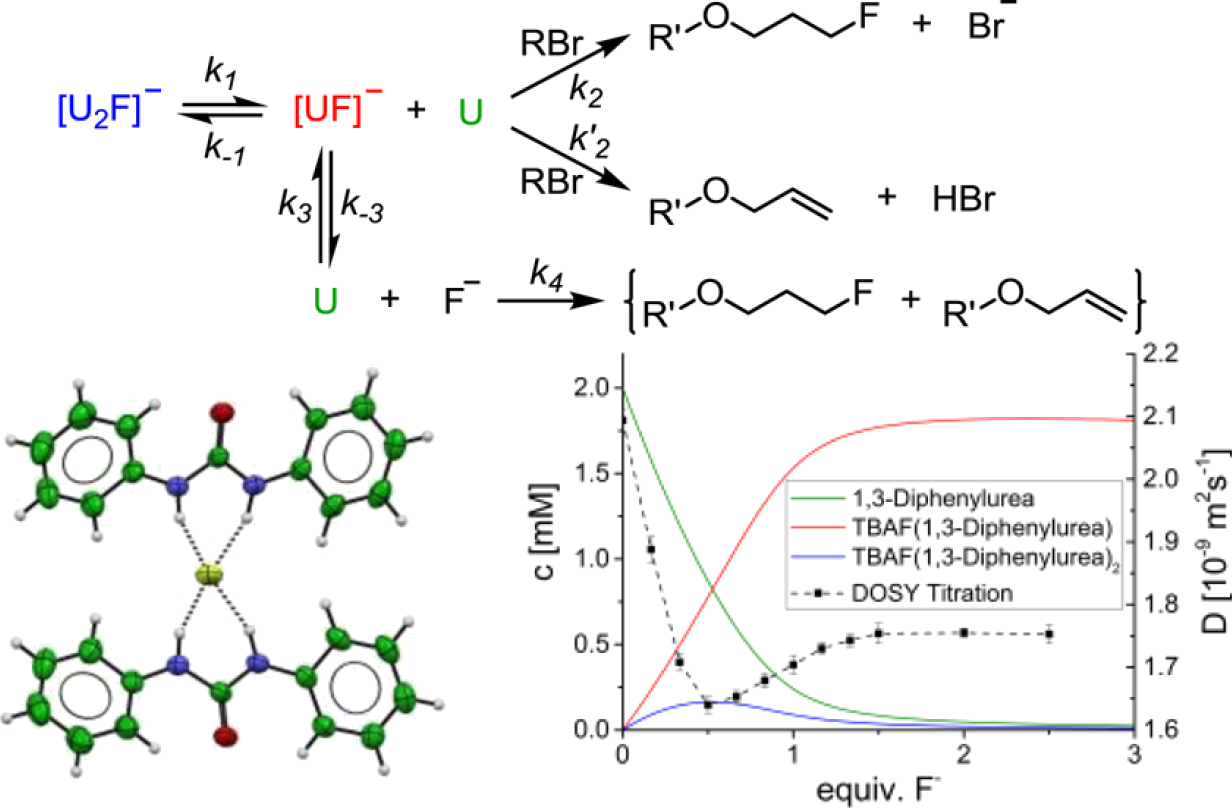
Hydrogen bonding with fluoride is a key interaction encountered when analyzing the mode of action of 5′-‚Äčfluoro-‚Äč5′-‚Äčdeoxyadenosine synthase, the only known enzyme capable of catalyzing the formation of a C-‚ÄčF bond from F–‚Äč. Further understanding of the effect of hydrogen bonding on the structure and reactivity of complexed fluoride is therefore important for catalysis and numerous other applications, such as anion supramol. chem. Herein we disclose a detailed study examg. the structure of 18 novel urea-‚Äčfluoride complexes in the solid state, by X-‚Äčray and neutron diffraction, and in soln. phase and explore the reactivity of these complexes as a fluoride source in SN2 chem. Exptl. data show that the structure, coordination strength, and reactivity of the urea-‚Äčfluoride complexes are tunable by modifying substituents on the urea receptor. Hammett anal. of aryl groups on the urea indicates that fluoride binding is dependent on ŌÉp and ŌÉm parameters with stronger binding being obsd. for electron-‚Äčdeficient urea ligands. For the first time, defined urea-‚Äčfluoride complexes are used as fluoride-‚Äčbinding reagents for the nucleophilic substitution of a model alkyl bromide. The reaction is slower in comparison with known alc.-‚Äčfluoride complexes, but SN2 is largely favored over E2, at a ratio surpassing all hydrogen-‚Äčbonded complexes documented in the literature for the model alkyl bromide employed. Increased second-‚Äčorder rate consts. at higher diln. support the hypothesis that the reactive species is a 1:1 urea-‚Äčfluoride complex of type [UF]–‚Äč (U = urea) resulting from partial dissocn. of the parent compd. [U2F]‚Äč–‚Äč. The dissocn. processes can be quantified through a combination of UV and NMR assays, including DOSY and HOESY analyses that illuminate the complexation state and H-‚Äčbonding in soln.
Publisher’s copy