A-level students from a range of schools attended a one-day course at the Museum of the History of Science and the Department of Chemistry in Oxford to find out about the science and applications of crystallography. In the morning they discovered how symmetry plays important role in the structure and diffraction of crystals in a lecture by Prof. Brian Sutton of King’s College, London. Prof. Richard Cooper then gave an rapid overview of the history of the applications of crystallography from Pasteur’s discovery of chirality in the pre- X-ray diffraction world to Hodgkin’s determination of the structure of penicillin. Prof. Elspeth Garman took the students through the ups and downs of crystallographic research in the decades long attempt to grow one crystal of a virus protein in an attempt to fight the tuberculosis virus.
A-level students from a range of schools attended a one-day course at the Museum of the History of Science and the Department of Chemistry in Oxford to find out about the science and applications of crystallography. In the morning they discovered how symmetry plays important role in the structure and diffraction of crystals in a lecture by Prof. Brian Sutton of King’s College, London. Prof. Richard Cooper then gave an rapid overview of the history of the applications of crystallography from Pasteur’s discovery of chirality in the pre- X-ray diffraction world to Hodgkin’s determination of the structure of penicillin. Prof. Elspeth Garman took the students through the ups and downs of crystallographic research in the decades long attempt to grow one crystal of a virus protein in an attempt to fight the tuberculosis virus.
Split into groups, the students then visited the Department of Chemistry where they visited three different activities:
- Rapid collection of diffraction data (under 10 minutes) and solution of the structure of fructose crystals with Dr. Amber Thompson in the X-ray facility.
-  Tasting how different crystalline structures (polymorphs) of cocoa butter in chocolate affect its texture and physical properties with Ms. Rachel Knight from Dirk Aarts’ research group (honorable mention to the one student who resisted temptation – having given up chocolate for lent!).
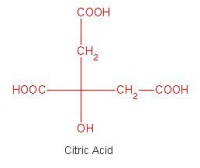
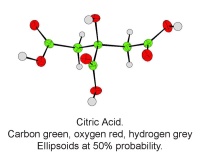
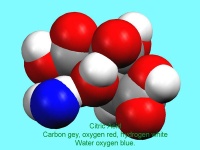
- Exploring stereoisomers and enantiomers using physical models (including Pasteur’s tartrate ion) and discovering why mirror images of a molecule can have quite different smells.
Meanwhile back in the museum students visited the solar fuels outreach stand where they saw how crystallography can reveal the structures that nature uses to carry out photosynthesis, and, under the careful supervision of Johnny Brooks-Bartlett and Katharina Jungnickel from Biochemistry, they were able to carry out a recrystallisation of the protein lysozyme and watch while it grew in just a few minutes on a microscope slide.