Presented by:Â Matthew J. Langton, Jonathan D. Matichak & Dr. Amber L. Thompson
Research Leader:Â Prof. Harry L. Anderson
Published:Â Chemical Science (cover article)
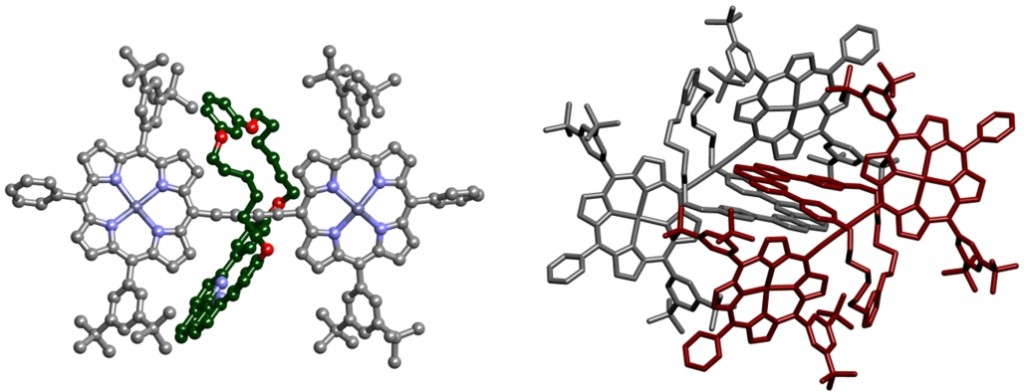
Fully π-conjugated porphyrin oligomers exhibit remarkable properties such as ultrafast energy migration, strong two-photon absorption and wire-like charge transport. The possibility to encapsulate them by rotaxane formation may provide valuable control over their properties by offering a unique approach to engineering intermolecular interactions. An active-template Cu-mediated Glaser coupling provides an efficient route to these structures. Data from crystals of this rotaxane were collected in-house. The porphyrin dimer is slightly twisted and non-linear, contrasting with previously reported structures which possess an inversion centre (and are thus rigorously planar). The phenanthroline units of the threaded macrocycle form tightly packed π-stacks in the crystal.