Dalton Trans. (2012), 41, 1951-1960. [ doi:10.1039/C1DT11758K ]
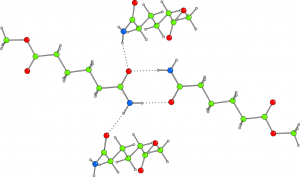
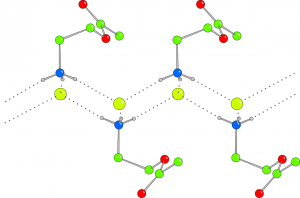
Uranium complexes of bis(p-tert-butyl-salicylidene)-1,2-diphenylethylenediamine (1) and bis(salicylidene)-1,2-diphenylethylenediamine (2) have been synthesized and investigated by X-ray single crystal diffraction and MD calculations in Periodic Boundary Conditions. Both compounds form crystals which are densely packed and do not provide voids accessible to solvent molecules. The configurations adopted by 1 and 2 are determined by well defined T-shaped and π-stacking non covalent interactions between phenyl groups of adjacent molecules as well as by a network of hydrogen bonds. These interactions and the relative arrangements of the molecules, explain the packing in the crystal structures. Each uranyl moiety shows a penta-coordination in the equatorial plane perpendicular to the trans oxygens giving rise, in both compounds, to a bypiramidal geometry. As usual for this class of compounds, the 5th position is characterized by the presence of the coordinated solvent. The in silico simulations confirm this hypothesis in very fine details. Moreover, in 1, even the partial occupancy of the solvent molecule determined from the crystal structure refinement, was shown to be due to a constrained freedom of motion of the solvent molecule that can be reproduced by molecular dynamics. This suggests that the reported disorder is not due to a poor quality of the harvested crystals but to a structural feature. In further agreement with the above mentioned results, DFT calculations demonstrated that the molecular orbital configuration and energies suit the described properties of complexes 1 and 2 suggesting a potential enantioselective activity as already shown by molecules belonging to this class of compounds.
Electronic reprints
- Oxford University Research Archive [direct pdf]
Publisher’s copy